As a company focusing on Human Platelet Lysate (HPL) development and supply for many years, we have a rich knowledge base. On this page, we want to show you an inside look into our ELAREM™ Human Platelet Lysate Technology.

ELAREM™ Technology
HUMAN PLATELET LYSATE TECHNOLOGY
ELAREM™ – A SUPERIOR TECHNOLOGY TO PRODUCE SAFE & EFFICIENT CELL CULTURE SUPPLEMENTS

WHAT IS THE ELAREM™ PLATFORM?
Our ELAREM™ platform is a portfolio of cell culture media based on human platelets. Due to their high growth factor content, human platelets are rich in nutrients necessary for in vitro cell expansion. The solution that extracts these relevant factors out of human platelets is called Human Platelet Lysate.
All our ELAREM™ products are xeno-free cell culture supplements. This means that the cell culture supplements and potential related products do not contain any animal components.
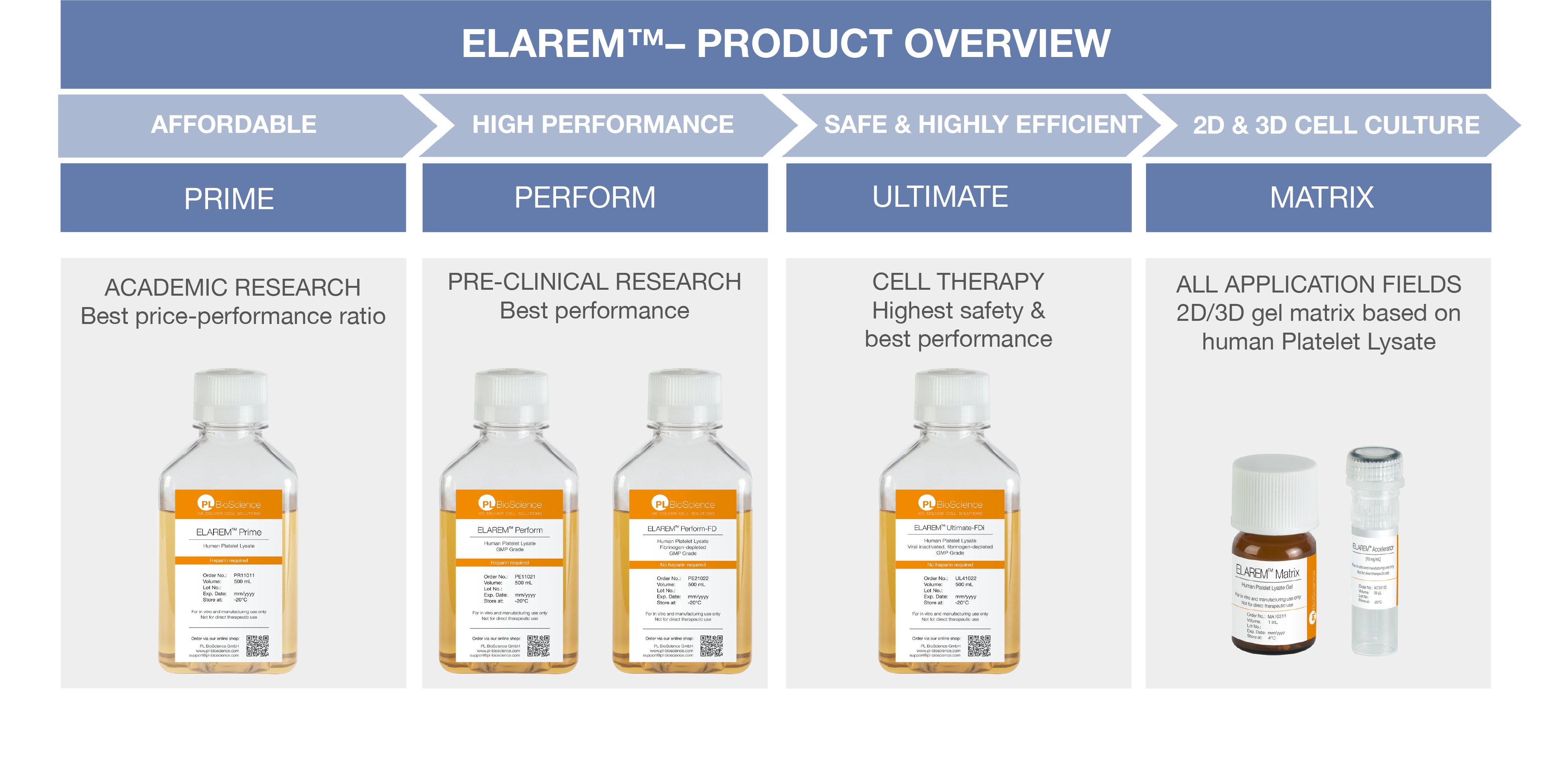
ELAREM™ – ONE SOLUTION AT A TIME
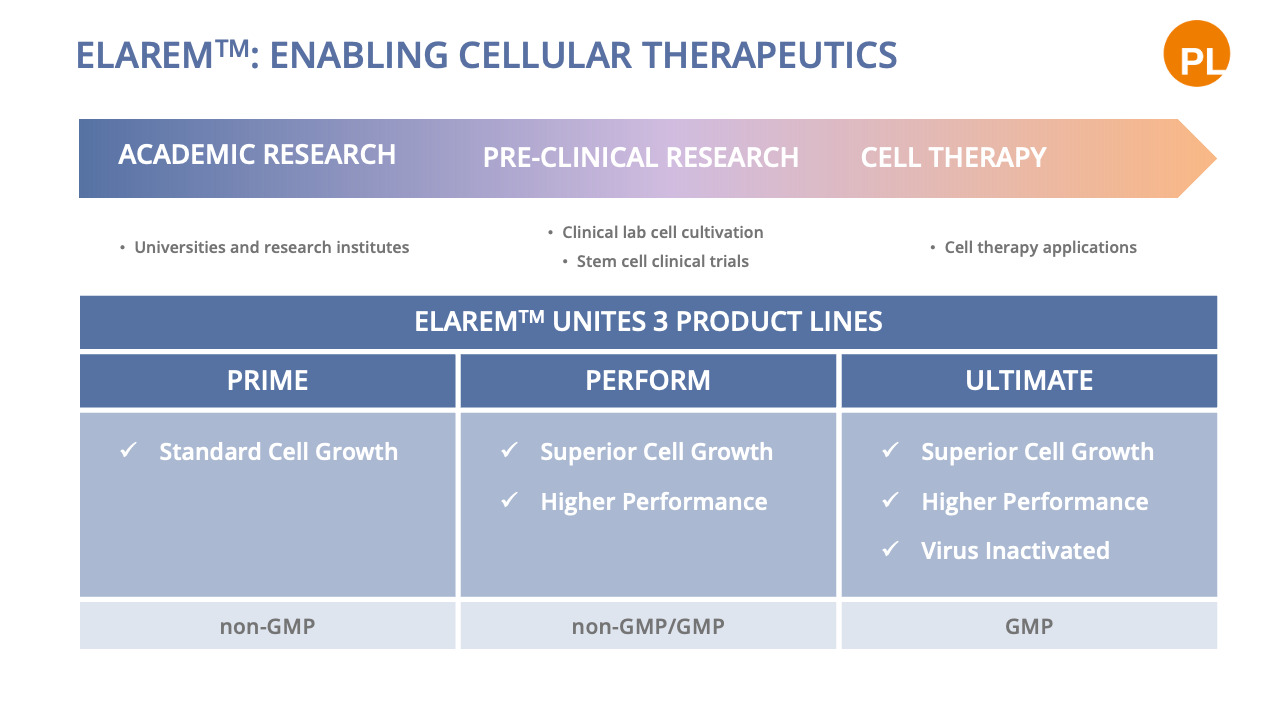
With our ELAREM™ Human Platelet Lysate Technology, we have created cell culture supplements that are able to support researchers across the whole research chain; With tailored cell culture supplements covering all needs of cell expansion from academic research, to pre-clinical research, and GMP-compliant cellular therapy.
ELAREM™ PRIME
ELAREM™ Prime is the worldwide first affordable cell culture supplement based on human platelets offering the same benefits as FBS – in an animal serum-free environment.
ELAREM™ PERFORM
ELAREM™ Perform is a Human Platelet Lysate that meets the needs of research and development. The safe and performance-increasing cell growth promoter ensures efficient lab processes and a xeno-free cell culture environment.
ELAREM™ Perform-FD and ELAREM™ Perform-FD PLUS are additionally fibrinogen-depleted and do not require anticoagulant addition.
ELAREM™ ULTIMATE
ELAREM™ Ultimate-FD PLUS and ELAREM™ Ultimate-FDi are gamma irradiated and fibrinogen-depleted Human Platelet Lysates. The products are suited to cell manufacturing needs requiring proven viral reduction, e.g. for potential use in clinical trials. Growth factors and cytokines are preserved and ensure cell culture performance for industrial in vitro cell expansion.
ELAREM™ MATRIX
ELAREM™ Matrix Kit provides a patent-protected gel scaffold based on Human Platelet Lysate. The system is suitable for 2D and 3D cell culture applications. The animal component-free technology combines both a nutrient source and a viscous scaffold in one – resembling the natural cell environment.
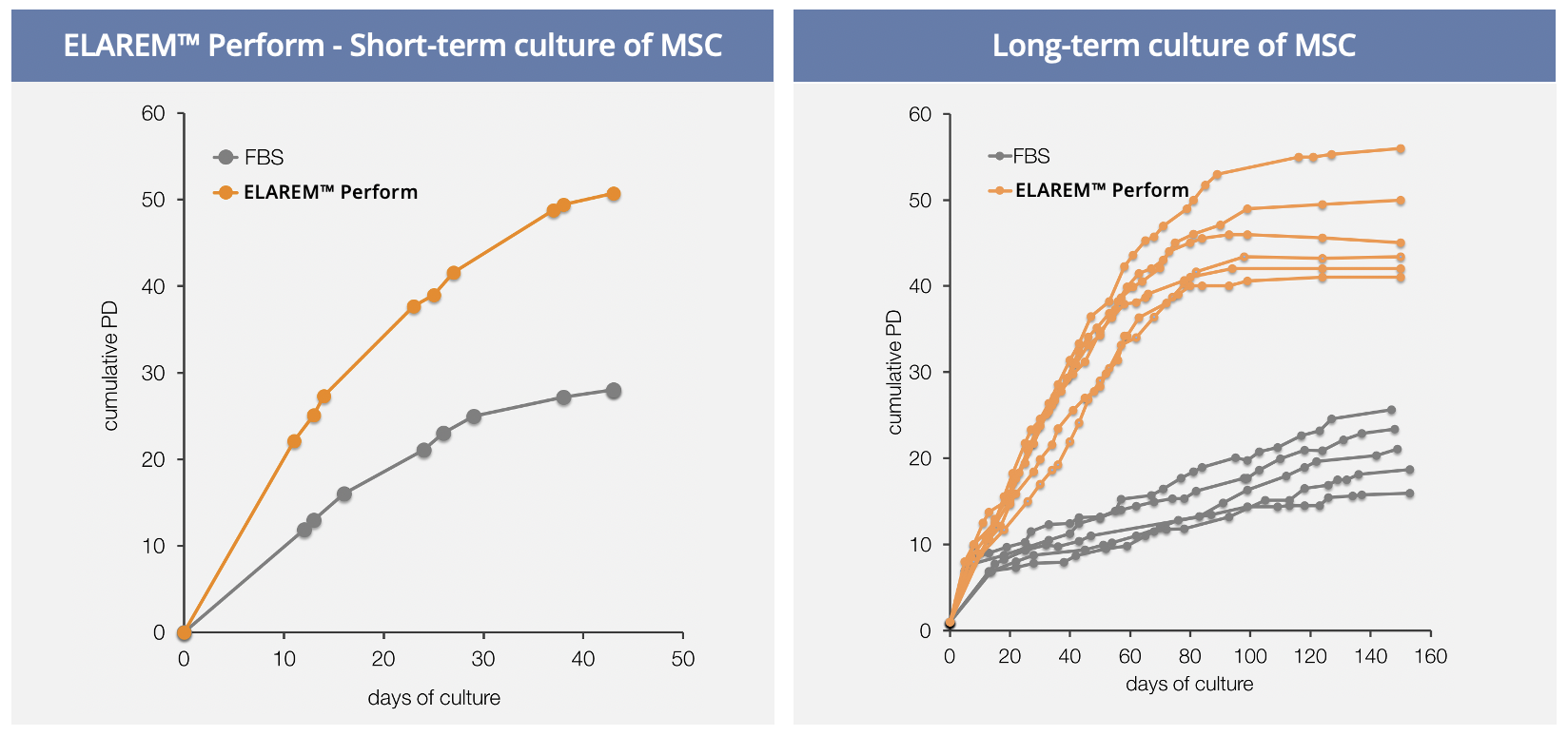
PERFORMANCE OF HUMAN PLATELET LYSATE
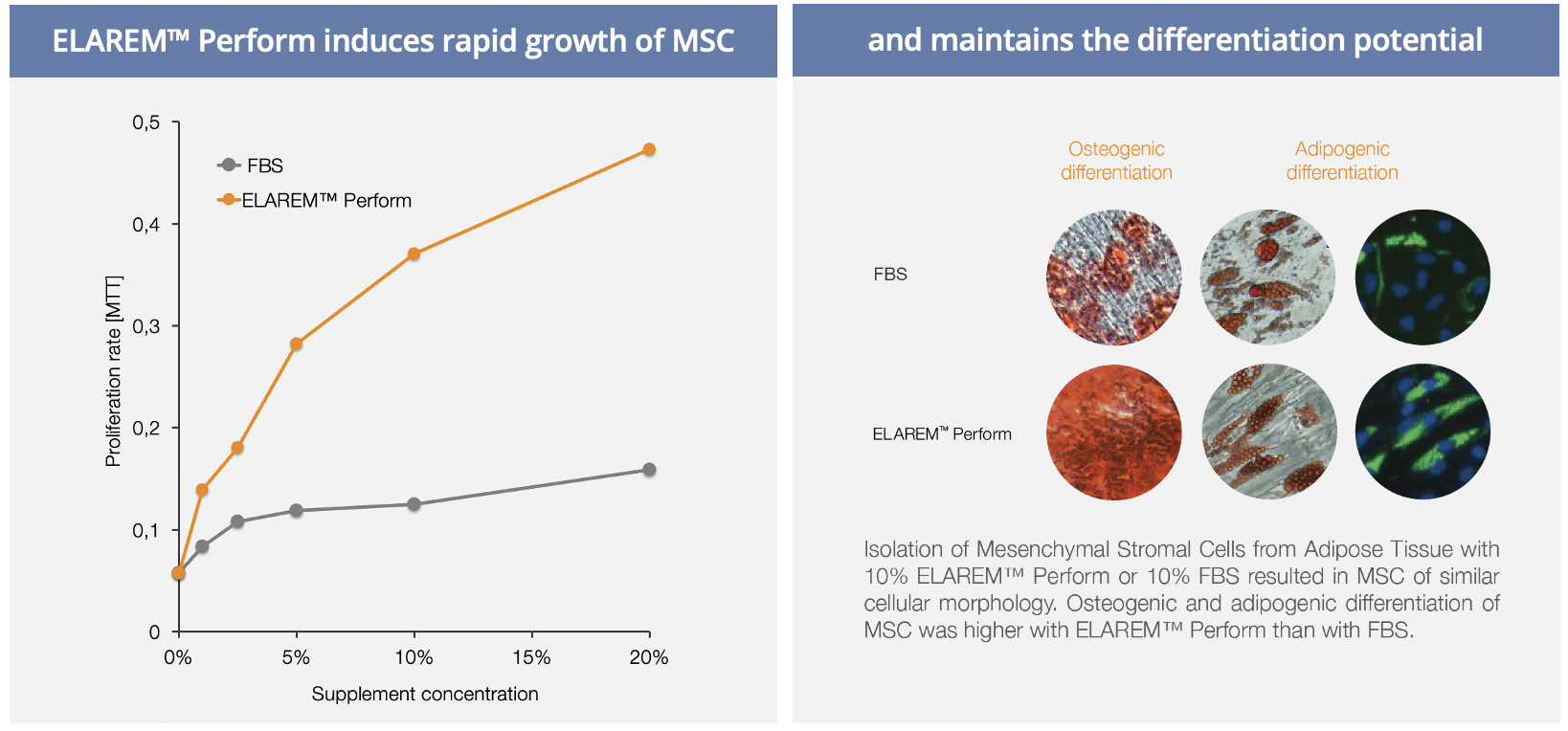
Human Platelet Lysate contains abundant growth factors and cytokines derived from human platelets. Those nutrients have been shown to stimulate cellular proliferation and maintain phenotype and differentiation potential of various cells.
ELAREM™ SUPPORTS THE IN VITRO EXPANSION OF VARIOUS PRIMARY CELLS AND CELL LINES
Our Human Platelet Lysates can be used for a numerous amount of cell types.
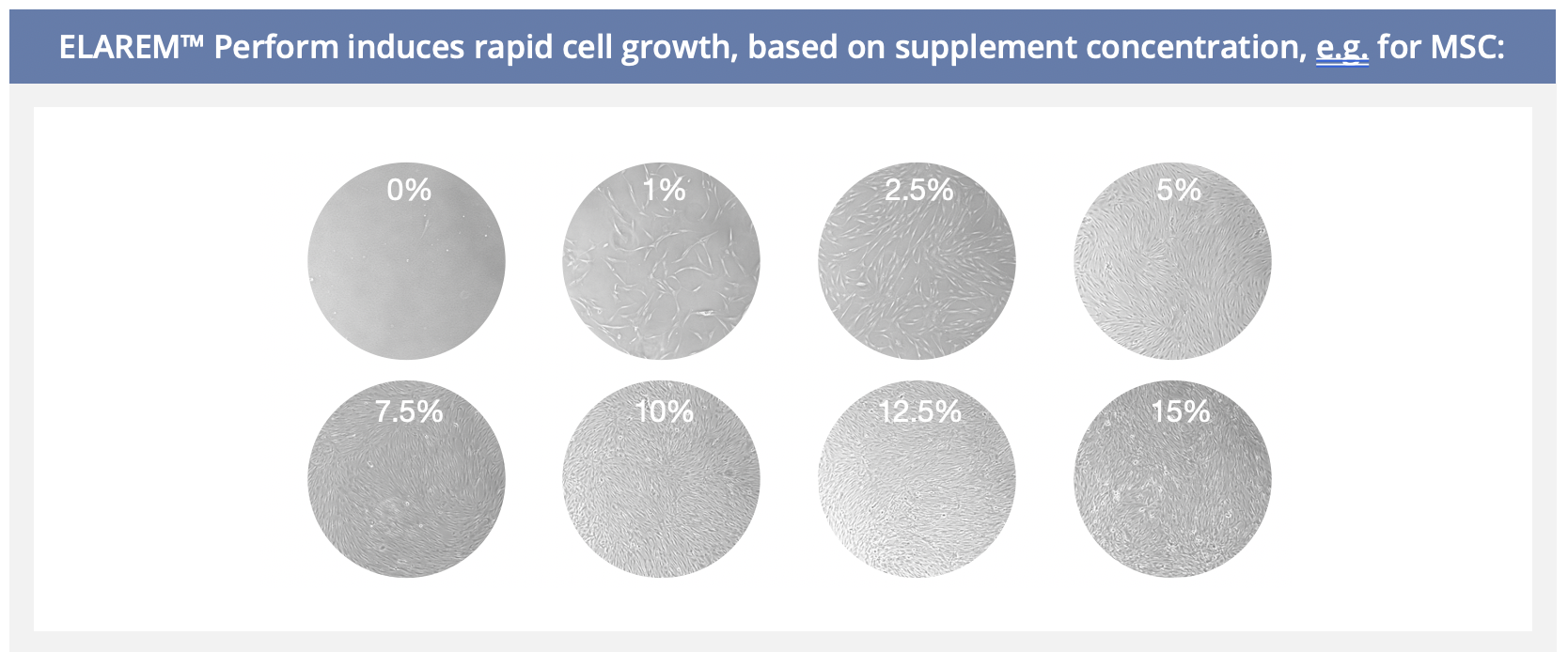
To achieve high cell proliferation rates, we recommend adding 10% (v/v) ELAREM™ Human Platelet Lysate to the complete cell culture medium.
Final concentration of ELAREM™ Human Platelet Lysate in cell culture medium can vary depending on cell type and experimental conditions. It is recommended to determine the optimal concentration – between 1% and 10% (v/v) – for the cells of interest.
A small extract of cells that profit from the nutrient-rich in vitro environment provided by human Platelet Lysate:
- Mouse Myoblastoma Cells
- Human continuous tumor cells
- Keratinocyte cell line from adult human skin (HaCaT)
- Human Embryonic Kidney 293 cells
- Cervical cancer cells (HeLa)
- Dermal fibroblasts
- Human leukemia cell lines
- Human umbilical vein endothelial cells
- Mesenchymal Stem Cells
- Dermal keratinocytes
- Vero cells
Based on a various number of publications and customer feedbacks, we have gathered data on a huge amount of cell types cultivated with Human Platelet Lysates like our ELAREM™ Human Platelet Lysate Technology. Please contact us if you have any specific inquiries or questions.

TIME & MONEY SAVING
The high concentration of growth factors and proteins accelerates cell proliferation, leading to faster results with less medium supplementation.

SAFETY
In addition to being free from ethical concerns, Human Platelet Lysate allows for much better traceability and does not pose a risk of animal-derived pathogen and prion transmission.

REPRODUCIBILITY
Our ELAREM™ Human Platelet Lysate ensures a high lot-to-lot consistency. This eliminates the need of FBS-related batch testing and ensures reproducible results – while human Platelet Lysate is suitable for human or animal primary cells and cell lines.

HOW IS HUMAN PLATELET LYSATE PRODUCED?
Human Platelet Lysate is derived from transfusion-approved human platelets (thrombocytes). Due to safety reasons, the storage capability of platelets required for blood transfusion is very limited. The platelets that cannot be used for blood transfusions any more are reprocessed to our cell culture medium. This represents a sustainable source that allows, unlike FBS, for independency from the slaughter industry.

RAW MATERIAL
Human platelet units are supplied by licensed blood donation centres where donors have been tested.

TRANSPORT
Platelet units need to be stored frozen within the entire transportation prior to manufacturing.

MANUFACTURING
Platelet units are subjected to a standardized manufacturing process. Raw materials, equipment and facilities are in compliance with applicable cGMP regulations.

FINAL PRODUCT
The final product is tested according to standardized validation tests while all criteria are documented.

CUSTOMER
The customers benefit from our look-back procedure enabling the tracing of every production step.
MANUFACTURING OF HUMAN PLATELET LYSATE
Quality and security is important when producing Human Platelet Lysate, therefore we produce our ELAREM™ Platform in qualified Controlled Environment Rooms (CERs) and cGMP compliant clean room areas.
PRODUCT REFERENCES
You find more references and publications on our publications page as well testimonials on our testimonials page.
Regulatory-compliant conditions during cell product manufacturing enhance in vitro immunomodulatory properties of infrapatellar fat pad-derived mesenchymal stem/stromal cells.
Kouroupis D, Bowles A, Greif D, et al. (2020). Cytotherapy.
View article
CD10/Neprilysin Enrichment in Infrapatellar Fat Pad-Derived Mesenchymal Stem Cells Under Regulatory-Compliant Conditions: Implications for Efficient Synovitis and Fat Pad Fibrosis Reversal.
Kouroupis D, Bowles A, Best T, et al. (2020). The American Journal of Sports Medicine, 1-15.
View article
Human Platelet Lysate Can Replace Fetal Calf Serum as a Protein Source to Promote Expansion and Osteogenic Differentiation of Human Bone-Marrow-Derived Mesenchymal Stromal Cells.
Karadjian M, Senger A-S, Essers C, et al. (2020). Cells; 9: 918.
View article